Projects
Exploring the Mechanisms of Immune Response
In the Reed lab, our goal is to study the mechanisms of immune responses and their downstream effects in conditions like the ones featured below. From there, we translate our findings to the clinic, developing targeted therapies for treatment and prevention of these conditions to better serve patients.
Our past and present funding sources include NIAID/NIH, NHLBI/NHI, W.M. Keck Foundation, Connie Frank and Evan Thompson Program for Collaborative Restorative Transplantation Research, Immucor, Inc., Novartis, and Bristol-Myers Squibb.
Antibody-Mediated Rejection (AMR)
HLA Signaling Through Endothelial Cell
Our lab studies graft loss through antibody mediated rejection (AMR), resulting in tissue vasculopathy (TV) and ultimately ischemic injury and graft rejection. Our research focuses on the role of donor specific antibodies and the response they illicit when binding to recipient HLA on endothelial cells. The role of the protein binding partners with HLA when bound by DSA and their mechanisms of action are being investigated to elucidate the activated endothelium and polarized macrophage phenotype seen in TV and graft rejection.

Figure Caption: Schematic explaining DSA-activated endothelium and macrophage polarization. HLA I antibody crosslinking on ECs forms a complex with Toll-like receptor 4 (TLR4), triggering TLR4 signaling via MyD88. This signaling leads to the exocytosis of Weibel-Palade bodies and surface expression of P-selectin. Monocytes then tether to ECs via PSGL-1-P-selectin and receive a secondary stimulus through FcγR-IgG interactions. Subsequently, monocytes adhere, undergo diapedesis, differentiate into polarized macrophages, and secrete vascular remodeling proteins that contribute to TV.
HLA YAP and TEAD Signaling Pathway in AMR
This project investigates the role of YAP/TEAD signaling in endothelial cell (EC) proliferation and migration in response to HLA donor-specific antibodies (DSAs) and explores the inhibitory effects of statins on this pathway. The study aims to (1) determine how HLA II antibody stimulation regulates YAP in human ECs, (2) elucidate the mechanism by which statins inhibit YAP function, EC proliferation, and migration following HLA I & II engagement, and (3) assess the impact of statins on YAP activity and chronic antibody-mediated rejection (cAMR) in a heart allograft model.
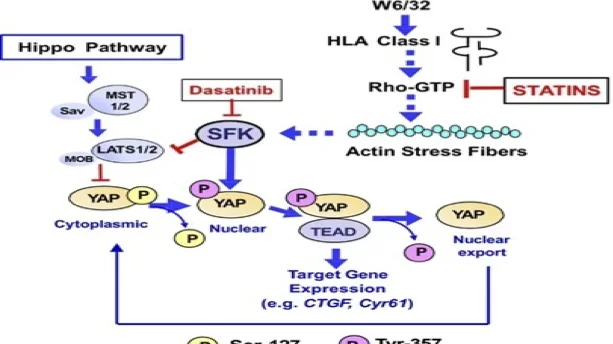
Figure Caption: Schematic of YAP regulation in endothelial cells via canonical Hippo and non-canonical HLA I pathways. The Hippo pathway phosphorylates YAP at Ser127, promoting cytoplasmic retention. HLA I stimulation (W6/32) activates Rho-GTP and actin stress fibers, promoting. YAP nuclear localization. SFKs phosphorylate YAP at Tyr357, enhancing TEAD-driven gene expression (CTGF, Cyr61). Dasatinib inhibits SFKs, while statins block Rho-GTP signaling, reducing YAP activity.
Ischemia-Reperfusion Injury
Ischemia Reperfusion Injury (IRI)
Ischemia-reperfusion injury (IRI) and organ preservation-related damage contribute to organ shortages and reduced allograft survival. In orthotopic liver transplantation (OLT), we investigate innate-adaptive immune crosstalk driving IRI, hypothesizing that identifying immune phenotypes will enable better monitoring and refinement of therapeutic interventions to improve transplant outcomes. Our work established an IRI scoring system, characterized cytokine profiles, and identified distinct pattern recognition receptor activation in IRI+ (TLR4, TLR9) vs. IRI- (TLR7, NOD2) patients, revealing disulfide HMGB1 as a key IRI driver. We now explore how disulfide HMGB1, mitochondrial DNA (mtDNA), and cell-free DNA (cfDNA) shape immune activation, the role of DAMPs and PRR signaling in IRI pathology, and whether immunologic memory to alloantigens exacerbates IR damage and generates alloreactive T cells that impact graft outcomes. Additionally, we are analyzing the dynamics of alloreactive T cells over time to understand their role in graft rejection and tolerance mechanisms. By integrating these longitudinal data on circulating immune cell phenotypes and alloreactive T cell responses, we aim to develop a comprehensive understanding of the immune responses involved in IRI and their implications for long-term transplant success.

Figure Caption: Schematic explaining the innate and adaptive immune cross-talk during liver transplantation. Upon liver transplantation, necrotic and injured cells release Damage-Associated Molecular Patterns (DAMPs), which activate Pattern Recognition Receptors (PRRs) on innate immune cells, epithelial, and endothelial cells. This activation triggers the production of inflammatory cytokines and chemokines, creating an inflammatory milieu. Macrophages process and present graft-derived antigens on their surfaces, which are then recognized by T-cells, activating the adaptive immune system. This interaction induces alloimmunity, potentially leading to graft rejection.
Systems Immunology
Cytomegalovirus Infection Transplant Recipients
The Human Immunophenotyping Project Consortium maps immune responses to Cytomegalovirus (CMV) in renal transplantation. CMV, a major threat to immunocompromised patients, increases the risk of graft dysfunction, loss, and mortality. The Reed Lab aims to map immune cross-talk in CMV infection, focusing on transplant rejection and injury. This will drive clinical innovations in vaccine strategies and risk assessment, ultimately reducing transplant-related injury.
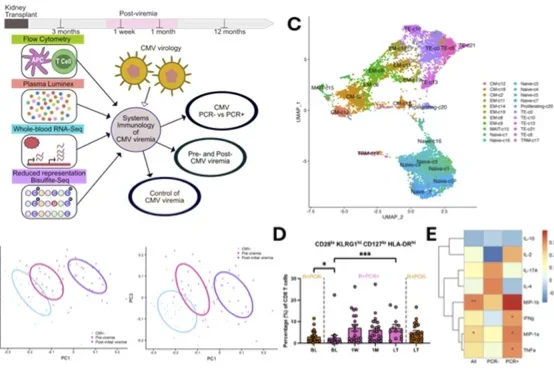
Figure Caption: Systems immunology of CMV summary figure. A) Study design. B) Partial least squares regression identifies immunological parameters as informative in defining stages of CMV viremia. C) Single-cell RNA sequencing of CD8+ T cells from KTRs identified 22 clusters. D) Frequency of CD8+ T cell cluster showing CD28lo KLRG1hi CD127lo HLA-DRhi differentiate R+ KTRs with and without CMV viremia before its detection at baseline (BL, 3mo-post Tx). E) Correlation of cluster frequency with CMV-stimulated PBMC cytokine and chemokine production.
Persistent Bloodstream Infections
Hematogenously disseminated candidiasis (HDC) is the most common cause of clinical persistent or resolving candidemia, and the mortality rate for this disease is unacceptably high despite treatment with appropriate antifungal therapies. Our main objective is to use systems biology to study the relationship between Candida pathogen-associated molecular patterns (PAMP)-induced innate immune reprogramming, epigenetic modification, T cell activation, and clinical outcomes of HDC. Additionally, in collaboration with Dr. Michael Yeaman of Harbor-UCLA, we focus on gaining detailed insights into the parallels between reprogramming of the host immune system by HDC vs MRSA.
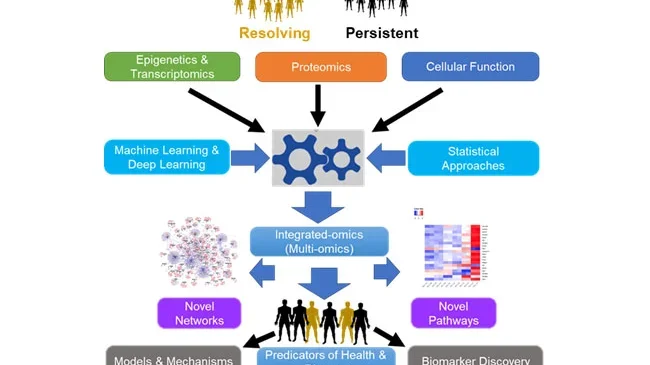
Figure Caption: A typical integrated omics workflow showing input datasets, output datasets and results. Using individual omics datasets that are closer to genotype (genomics and transcriptomics), and those closer to phenotype (proteomics and flowcytometry, datasets are integrated using statistical or advanced machine learning approaches. Results may be simple pathways or complex networks and may include both known and novel immune signatures. In addition, results may predict outcome of candidiasis into recurring or persistent disease states, provide insights for effective therapeutic interventions.
SARS-CoV-2 Infection and Vaccination
SARS-CoV-2 is the cause of COVID-19. The Reed Lab studies host immune responses to SARS-CoV-2 to improve vaccine design and delivery, focusing on limiting pathogen replication and preventing disease. We study participants in the LA-SPARTA cohort to understand the immune response to SARS-CoV-2 vaccination and infection. We also work with UC Davis' ADIVKT trial to assess vaccination response in immuno-suppressed transplant recipients. Through the NIAID-funded IMPACC study, we track immune responses in hospitalized COVID- 19 patients to predict disease severity. These studies use systems-based, high-throughput omics, and novel statistical approaches for a comprehensive assessment, aiming to improve clinical care, vaccine design, and delivery, especially in high-risk populations.
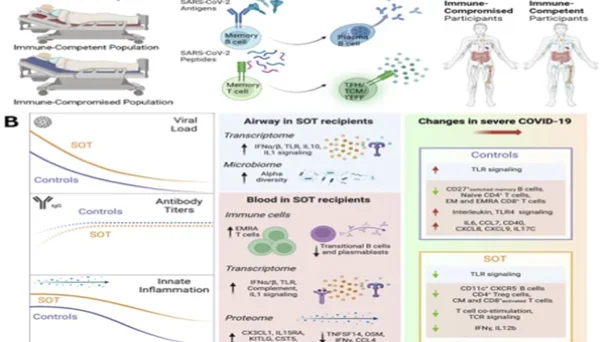
Figure Caption: Systems immunology of SARS-CoV-2 summary figure. A) Longitudinal analysis of SARS-CoV-2 infection and vaccination in the LA-SPARTA cohort reveals increased risk of infection in vaccinated Hispanic participants. B) Summary schematic highlighting inflammatory dysregulation in solid organ transplant recipients hospitalized for COVID-19 based on host/microbe multiomic profiling.