Projects
In the Reed lab, we study the mechanisms of immune responses and their downstream effects in multiple conditions such as antibody-mediated rejection (AMR) in multiple solid organ transplants, ischemia-reperfusion injury (IRI) in liver transplants, and peristant versus resolving viral and bacterial infections.
Our ultimate goal is to translate our findings to the clinic via development of targeted therapies for treatment and prevention of these conditions to better serve patients. Our past and present funding sources include NIAID/NIH, NHLBI/NHI, W.M. Keck Foundation, Connie Frank and Evan Thompson Program for Collaborative Restorative Transplanation Research, Immucor, Inc., Novartis, and Bristol-Myers Squibb.
Project 1: Antibody-Mediated Rejection
.jpg)
After successful solid-organ transplantation, nearly 50% of patients will eventually lose their grafts through a process called antibody-mediated rejection (AMR). This is mostly due to the polymorphic nature of a class of proteins called human leukocyte antigens, or HLA. Recipient immune cells recognize donor tissue as foreign due to disparities in HLA, and mount an immune response against the graft. This results in the production of donor-specific HLA antibodies, or DSA, which mediate rejection by activating the endothelium mediating the recruitment of circulating leukocytes and monocytes. Chronic inflammation results in thickening of the vascular wall, a process termed transplant vasculopathy (TV). TV results in vessel occlusion leading to ischemic injury and graft rejection.
DSA signal through donor HLA expressed by endothelium present in the graft causing endothelial cell activation and upregulation of P-selectin. In vitro studies in our lab have shown that antibody-activated endothelium skews monocytes to M2-like macrophages via interactions with a) adhesion molecules (e.g., P-selectin and PSGL-1) and b) IgG and Fc-receptors (FcyRs) during the process of transmigration.
Studies in our lab are also focused in determing the role of innate and adaptive immune cells in mediating TV. We utilize digital spatial profiling and multi-omics approaches to examine both protein and whole transcriptomic expression of vessels from DSA+ rejected cardiac allografts.
Recent Publications:
1. Salehi S, Sosa RA, Jin YP, Kageyama S, Fishbein MC, Rozengurt E,Kupiec-Weglinski JW, Reed EF. Outside-in HLA class I signaling regulates ICAM-1 clustering and endothelial cell-monocyte interactions via mTOR in transplant antibody-mediated rejection. American Journal of Transplantation. 2018 May;18(5):1096-1109.
2. Jin YP, Valenzuela NM, Zhang X, Rozengurt E, Reed EF. HLA Class II-Triggered Signaling Cascades Cause Endothelial Cell Proliferation and Migration: Relevance to Antibody-Mediated Transplant Rejection. Journal of Immunology. 2018 Apr 1;200(7):2372-2390.
3. Valenzuela NM, Reed EF. Antibody-mediated rejection across solid organ transplants: manifestations, mechanisms, and therapies. Journal of Clinical Investigation. 2017 Jun 30;127(7):2492-2504.
4. Thomas KA, Valenzuela NM, Reed EF. The perfect storm: HLA antibodies, complement, FcγRs, and endothelium in transplant rejection. Trends in Molecular Medicine. 2015 May;21(5):319-29.
5. Zhang X, Rozengurt E, Reed EF. HLA class I molecules partner with integrin β4 to stimulate endothelial cell proliferation and migration. Science Signalling. 2010 Nov 23;3(149):ra85.
Project 2: Ischemia-Reperfusion Injury
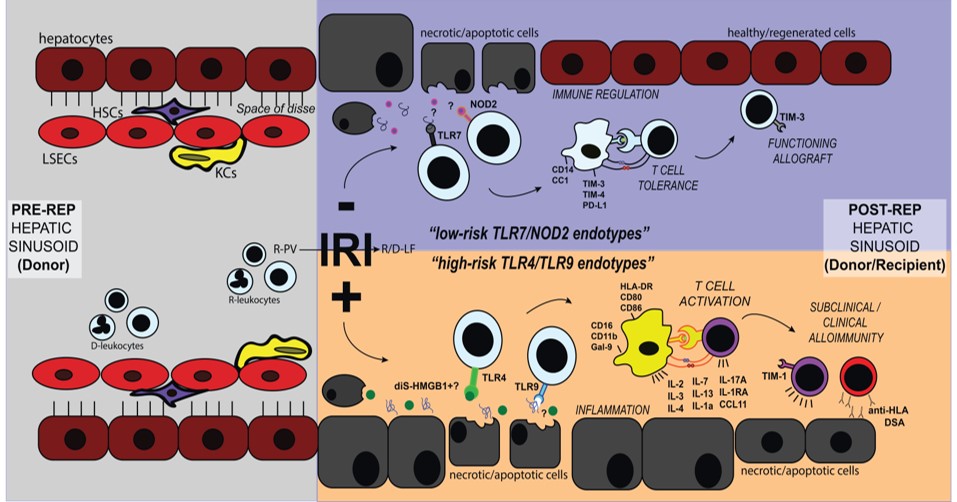
Cellular damage elicited by organ preservation and ischemia reperfusion injury (IRI) contributes to the organ shortage and lowers both short and long-term allograft survival. IRI is mediated by both the innate and adaptive immune systems. We study the crosstalk between innate and adaptive immunity in the context of IRI during orthotopic liver transplantation (OLT). Our central hypothesis that identifying the continuum of innate and adaptive immune phenotypes will permit us to select, monitor and refine the practice of therapeutic interventions against IRI and hence improve liver transplant outcomes. We have enrolled over 160 OLT patients for chronological immune assessment via peripheral blood protein analysis and immunophenotyping, RNAseq analysis of liver biopsies, and other in vitro assays, with the intentions of defining the underlying mechanisms controlling IRI in OLT.
Our published work includes an IRI scoring system, longitudinal cytokine profiles of IRI patients, and identification of pattern recognition receptors TLR4 and TLR9 activated in IRI+ vs TLR7 and NOD2 activated in IRI- patients. This led us to discovering that the disulfide form of the protein HMGB1 is important for driving IRI in OLT patients. We are currently exploring the mechanism by which disulfide HMGB1 impacts innate and adaptive immune activation post-OLT. We are also determining the role of other DAMPs and pattern recognition receptor signaling in this immune activation and delineating the pathological signature of human OLT IRI in allograft biopsies. Finally, we are determining the extent to which immunologic memory to allo-antigens augments IR-damage in OLT; and if IRI leads to the generation of alloreactive T cells that play a role in graft outcome.
Recent Publications:
1. Sosa RA, Rossetti M, Naini BV, Groysberg VM, Kaldas FM, Busuttil RW, Chang YL, Gjertson DW, Kupiec-Weglinski JW, Reed EF. Pattern Recognition Receptor-reactivity Screening of Liver Transplant Patients: Potential for Personalized and Precise Organ Matching to Reduce Risks of Ischemia-reperfusion Injury. Annals of Surgery. 2018 Nov 22.
2. Sosa RA, Zarrinpar A, Rossetti M, Lassman CR, Naini BV, Datta N, Rao P, Harre N, Zheng Y, Spreafico R, Hoffmann A, Busuttil RW, Gjertson DW, Zhai Y, Kupiec-Weglinski JW, Reed EF. Early cytokine signatures of ischemia/reperfusion injury in human orthotopic liver transplantation. Journal of Clinical Investigation Insight. 2016 Dec 8;1(20):e89679.
Project 3: Systems Immunology Approach for Infection
.jpg)
Persistant vs. Resolving Methicillin-resistant Staphylococcus aureus (MRSA) Infections
Methicillin-resistant Staphylococcus aureus (MRSA) infection is a major global healthcare problem. Of concern is antibiotic-persistent MRSA bacteremia (APMB), which can cause complicated invasive and metastatic infections such as infective endocarditis and organ abscesses. Moreover, persistent MRSA infections are refractory to antibiotic treatment in vivo, even though most such isolates are susceptible in in vitro to gold-standard agents such as vancomycin. The Reed Lab, in collaboration with Drs. Michael Yeaman of Harbor-UCLA and Vance Fowler at Duke University, focuses on profiling host signatures associated with APMB clinical outcomes. Our central hypothesis is that APMB results from a three-way interaction among the pathogen, host immune response and antibiotic. The Reed Lab employs systems-based statistical and computational immunology approaches to integrate results of high-throughput immunomics, genomics and transcriptomics data to model the pathogen-host signatures unique to APMB. This will allow our lab to identify host immune signatures associated with APMB that may provide predictive indicators applicable to clinical decision-making for improved treatment outcomes.
Recent Publications:
1: Chan LC, Rossetti M, Miller LS, Filler SG, Johnson CW, Lee HK, Wang H, Gjertson D, Fowler VG Jr, Reed EF, Yeaman MR; MRSA Systems Immunobiology Group. Protective immunity in recurrent Staphylococcus aureus infection reflects localized immune signatures and macrophage-conferred memory. Proceedings of the National Academy of Sciences of the United States of America. 2018 Nov 20;115(47):E11111-E11119.
Cytomegalovirus (CMV) Infection in Transplant Patients
Cytomegalovirus (CMV), a betaherpesvirus, has evolved alongside humans for thousands of years with a complex balance of latency, immune evasion, and transmission. While up to 70% of humans worldwide have evidence of CMV infection and seroprevalence approaches 100% in certain areas, healthy people show little to no clinical symptoms of primary infection. However, CMV is one of the most problematic pathogens in the immunocompromised host, after solid organ and stem cell transplantation causing increased risk of graft dysfunction, graft loss and recipient mortality. Dr. Reed leads the UCLA research team in the Human Immunophenotyping Project Consortium project focused on mapping immune responses to CMV in renal transplantation. Using a systems biology approach, carefully curated clinical information and novel statistical and computational approaches we will develop a detailed molecular map of the cross-talk between the innate and adaptive immune response in primary and latent CMV infection in the transplant recipient in order to define the effects of CMV infection on the development of transplant rejection and, more broadly, injury. Detailed insights into the interaction of the virus with the immune system stand to generate new concepts for more adequate vaccine strategies and risk assessment for CMV infection, and possibly for reduced or delayed immune injury to the transplanted organ.
Recent Publications:
1. Schaenman JM, Rossetti M, Sidwell T, Groysberg V, Sunga G, Korin Y, Liang E, Zhou X, Abdalla B, Lum E, Bunnapradist S, Pham T, Danovitch G, Reed EF. Increased T cell immunosenescence and accelerated maturation phenotypes in older kidney transplant recipients. Human Immunology. 2018 Sep;79(9):659-667.
2. Bakir M, Jackson NJ, Han SX, Bui A, Chang E, Liem DA, Ardehali A, Ardehali R, Baas AS, Press MC, Cruz D, Deng MC, DePasquale EC, Fonarow GC, Khuu T, Kwon MH, Kubak BM, Nsair A, Phung JL, Reed EF, Schaenman JM, Shemin RJ, Zhang QJ, Tseng CH, Cadeiras M. Clinical phenomapping and outcomes after heart transplantation. The Journal of Heart and Lung Transplantation. 2018 Mar 22. pii: S1053-2498(18)31391-3.
3. Liang EC, Rossetti M, Sidwell T, Groysberg V, Sunga G, Korin Y, Vangala S, Abdalla B, Lum E, Bunnapradist S, Pham PT, Danovitch G, Reed EF, Schaenman J. Differences in Proinflammatory Cytokines and Monocyte Subtypes in Older as Compared With Younger Kidney Transplant Recipients. Transplantation Direct. 2018 Feb 14;4(3):e348.
4. Schaenman JM, Rossetti M, Korin Y, Sidwell T, Groysberg V, Liang E, Vangala S, Wisniewski N, Chang E, Bakir M, Bondar G, Cadeiras M, Kwon M, Reed EF, Deng M. T cell dysfunction and patient age are associated with poor outcomes after mechanical circulatory support device implantation. Human Immunology. 2018 Apr;79(4):203-212.
5. Roedder S, Sigdel T, Hsieh SC, Cheeseman J, Metes D, Macedo C, Reed EF, Gritsch HA, Zeevi A, Shapiro R, Kirk AD, Sarwal MM. Expression of Mitochondrial-Encoded Genes in Blood Differentiate Acute Renal Allograft Rejection. Frontiers in Medicine. 2017 Nov 1;4:185.
6. Schaenman JM, Korin Y, Sidwell T, Kandarian F, Harre N, Gjertson D, Lum EL, Reddy U, Huang E, Pham PT, Bunnapradist S, Danovitch GM, Veale J, Gritsch HA, Reed EF. Increased Frequency of BK Virus-Specific Polyfunctional CD8+ T Cells Predict Successful Control of BK Viremia After Kidney Transplantation. Transplantation. 2017 Jun;101(6):1479-1487.
Systems Immunology of Viral Infection and Vaccination
Vaccination is the primary approach for controlling viral infections, however, we lack an effective vaccine for many viruses and existing vaccines are variably efficacious To improve vaccine design and delivery, it is important to understand host responses to infection and how they limit pathogen replication and prevent pathological outcomes Through the NIAID funded Center for Influenza Vaccine Research for High Risk Populations (CIVR HRP) study, the Reed lab is involved in research on protective host responses to provide insight into the mechanisms that drive influenza vaccine effectiveness in the most vulnerable, high risk populations. As part of this collaboration, the Reed lab is also studying a cohort of participants in the Los Angeles SARS-CoV-2 SeroPrevalence and Respiratory Tract Assessment observational study (LA-SPARTA) to gain insight into the immune response to SARS-CoV-2 vaccination and natural infection. The Reed lab also collaborates with UC Davis’ ADIVKT trial group to assess the response to SARS-CoV-2 vaccination in immune suppressed renal transplant patients. Additionally, through the NIAID funded IMMuno Phenotyping Assessment in a COVID 19 Cohort (IMPACC) study, the Reed lab is helping to track the immune responses of people hospitalized with COVID 19 to determine how certain immunological measures correspond to, or may even predict, the clinical severity of disease These highly innovative studies apply systems based, high throughput omics and novel statistical approaches to provide a comprehensive longitudinal assessment of the host response to natural infection and vaccination, aiming to improve clinical care, vaccine design and delivery, particularly in high risk populations.